SYN006(B+P)
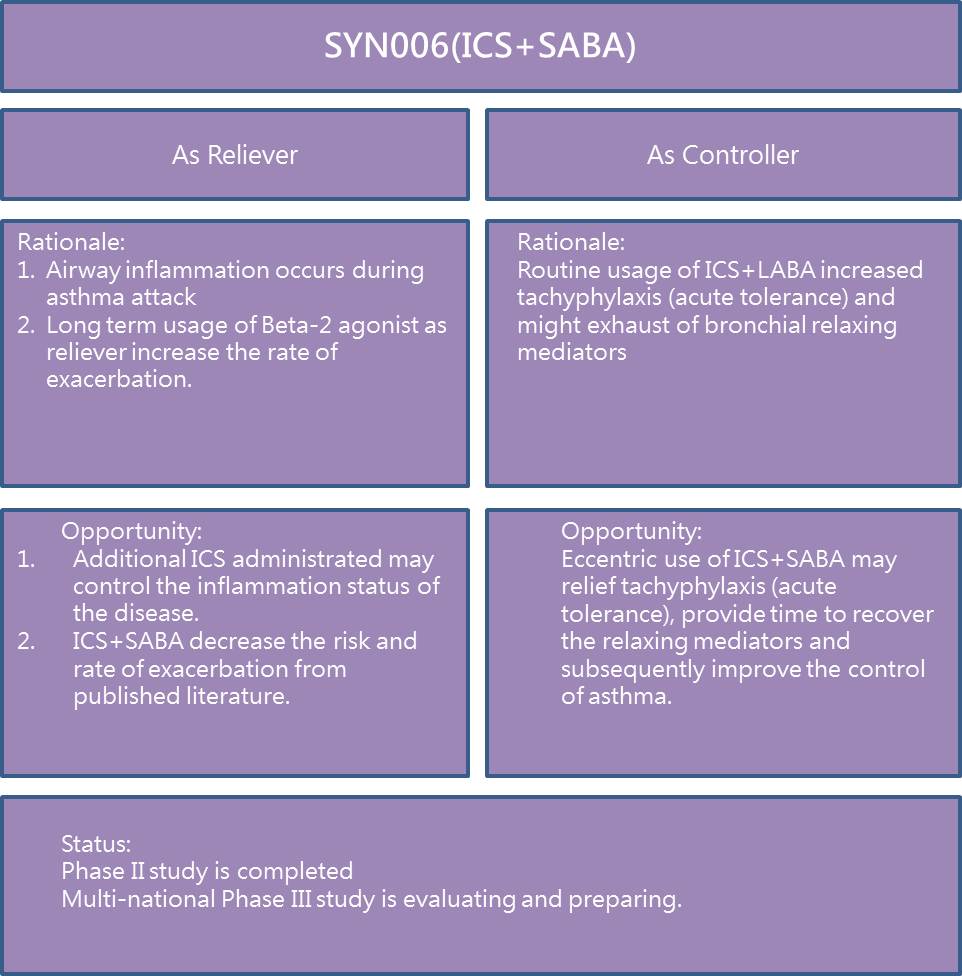
SYN006 HFA MDI is a unique combinational product with inhaled corticosteroid and short acting beta-2 agonist (ICS+SABA). A Phase II dose response study is completed in 2014 and multi-national Phase III studies are preparing for submission. The product is primarily used against emergency treatment (reliever) to relief the bronchoconstriction symptoms when asthma attacks. In addition, it can also be used as maintenance therapy which might improve the drug resistance issues of existing drugs for asthmatic patients. Patent of pharmaceutical combination has been granted from US, China, Canada, Australia, New Zealand, South Korea and Hong Kong. Applications for other countries and regions are also under reviewing.